The bond dipole is modeled as δ δ with a distance d between the partial charges δ and δ It is a vector parallel to the bond axis pointing. We would like to show you a description here but the site wont allow us.
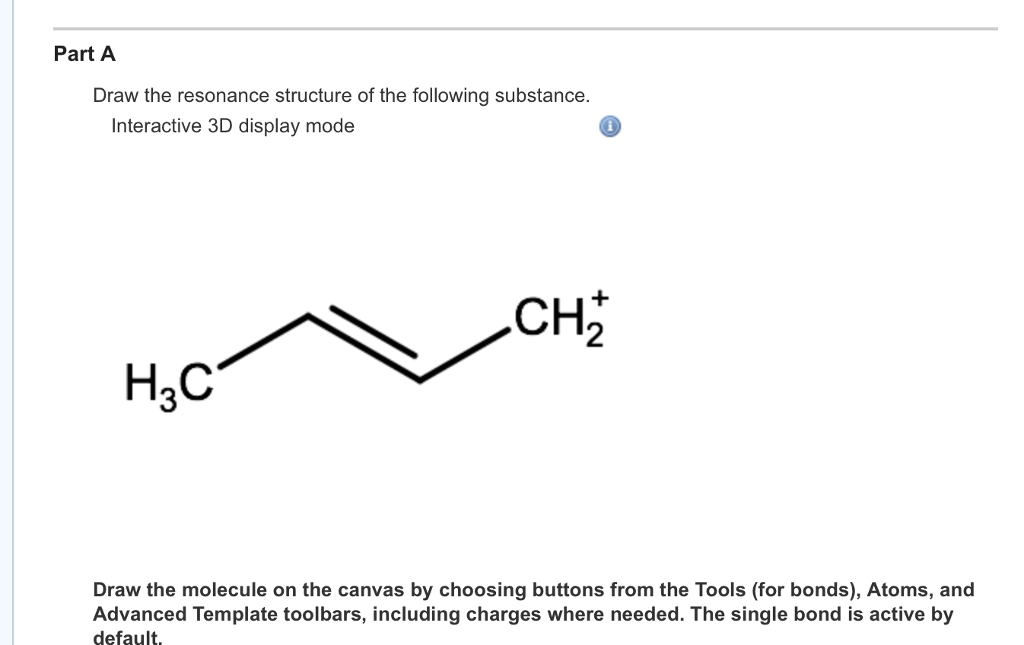
Solved Part A Draw The Resonance Structure Of The Following Chegg Com
The relative strength of the four intermolecular forces.
. For each of the following compounds draw the Lewis structure and orbital structure. Compare the different butane alcohol derivatives shown below. Molecular Structure of CBD.
The differences here include the number of oxygens attached to nitrogen. All the atoms attached to the middle atom are identical. The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a moleculeIt occurs whenever there is a separation of positive and negative charges.
The structure of DNA is dynamic along its length being capable of coiling into tight loops and other shapes. Draw the structures of the monomers from a section of the polymer. Use the References to.
A resonance hybrid is a composite of all resonance structures that spreads out electron pairs in multiple bonds and lone pairs. So polar equivalent is Transcribed image text. Explain the nature of the intermolecular forces between molecules of condensation polymers.
Based on the N-O bond distances of 1169 and 1442 pm on HNO_2 I would expect the bond length to be between 1169 and 1442 pm perhaps around 125 greater than 106. The amount of heat involvedQ in heating any substance is given by the relation- Q mSΔT where m. Be sure to label the sigma and pi bonds and place electrons in.
The bond dipole μ is given by. Look at a review of acids then dive into. Either A T C or G.
Notice the bonding patterns. In all species it is composed of two helical chains bound to each other by hydrogen bondsBoth chains are coiled around the same. DNA is a long polymer made from repeating units called nucleotides each of which is usually symbolized by a single letter.
Draw the repeating unit from monomer structures draw the repeating unit from a section of the polymer chain. Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds. CBD is a terpenophenol compound containing twenty-one carbon atoms with the formula C 21 H 30 O 2 and a molecular weight of 314464 gmol Figure 1The chemical structure of cannabidiol 2-1R-3-methyl-6R-1-methylethenyl-2-cyclohexen-1-yl-5-pentyl-13-benzenediol was determined in 1963 The current IUPAC.
A polar covalent bond would form in which one of the following pairs of atoms. Based on the N-O bond distances of 1199 1211 and 1406 pm I would estimate. The values of pH and pKa are directly affected by the molecular structures of acids and bases.
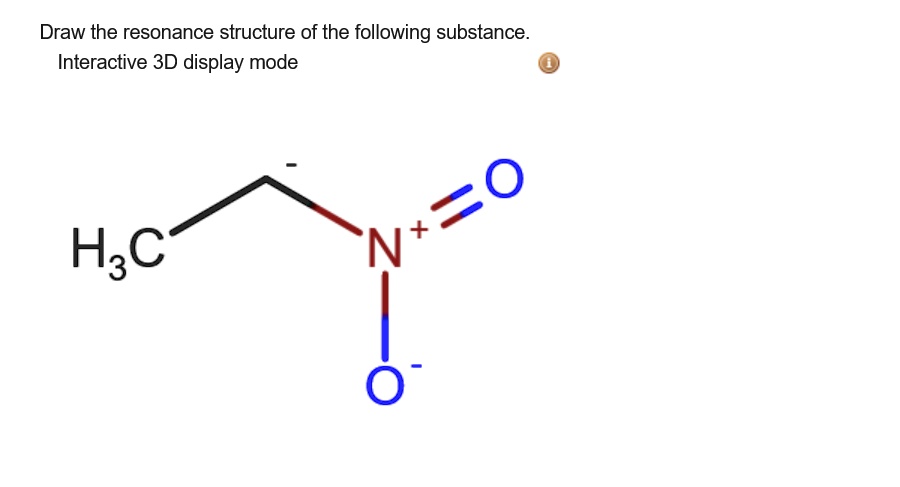
Solved Draw The Resonance Structure Of The Following Chegg Com

Draw The Resonance Structure Of The Following Substance Study Com
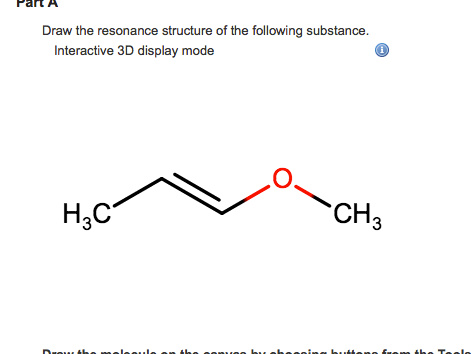
Solved Draw The Resonance Structure Of The Following Chegg Com
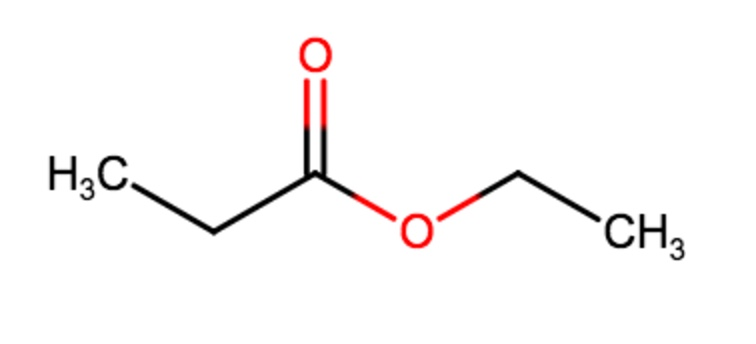
Solved Helppp Draw The Resonance Structure Of The Chegg Com
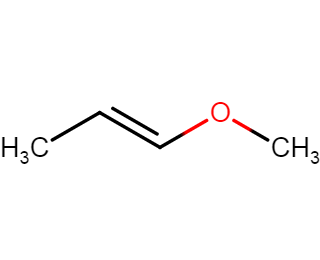
Solved Draw The Resonance Structure Of The Following Chegg Com

Solved Draw The Resonance Structure Of The Following Substance Interactive 3d Display Mode H3c N O
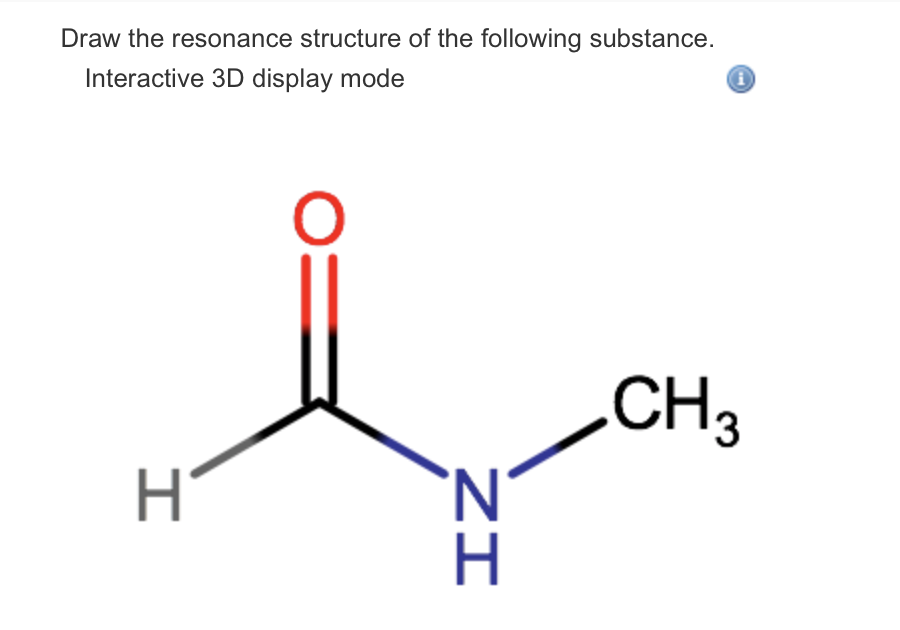
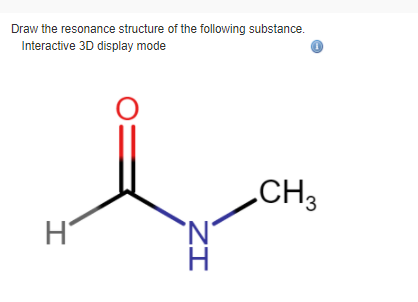
Solved Draw The Resonance Structure Of The Following Chegg Com

Draw The Resonance Structure Of The Following Substance Study Com
0 comments
Post a Comment